Conform FDA、CDE
Professional and High Quality
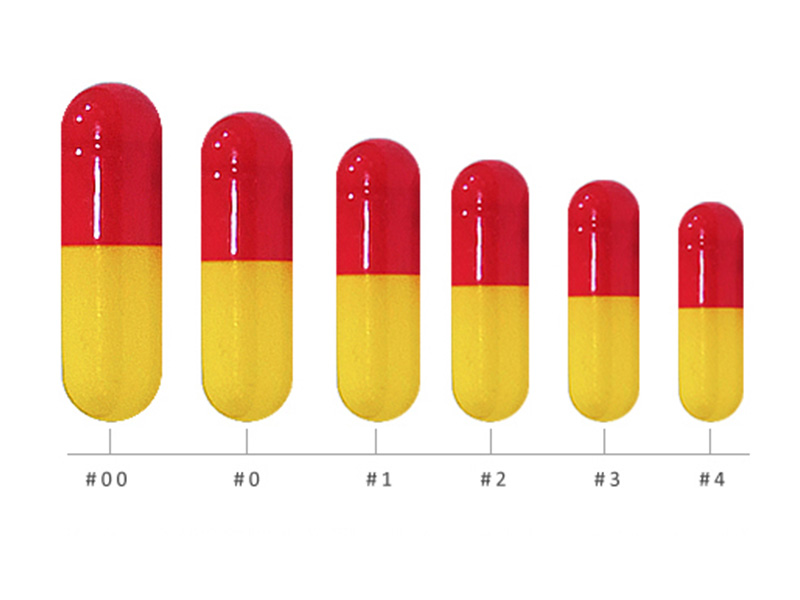
Shing Lih Fang has a long history of being a professional and excellent quality empty capsule manufacturer in Taiwan, we had continuously achieved the full steps of cGMP, PIC/S GMP standard, and obtained various international qualifications, we always have the professional confidence to provide the excellent quality empty capsules and efficient service to our customers.
In recent year and for future expansion, our gelatin capsules are also registered in U.S. FDA and CDE of China.
 HALAL
HALAL ISO9001
ISO9001 PIC/S GMP
PIC/S GMP China NMPA
China NMPA United States FDA
United States FDAExport registration number
United States DMF NO. 20588|China NMPA NO. F20180001281